FDA OKs Cell Therapy to Lower Infection Risk After Stem Cell Transplant
Por um escritor misterioso
Descrição
Omidubicel reduced infections in blood cancer patients from 60% to 39% at 100 days posttransplant

Review of 4 cell therapy types under study for COVID-19 - The Niche
FDA investigates risk of secondary malignancies with CAR T-cell

Systematic Review of Induced Pluripotent Stem Cell Technology as a
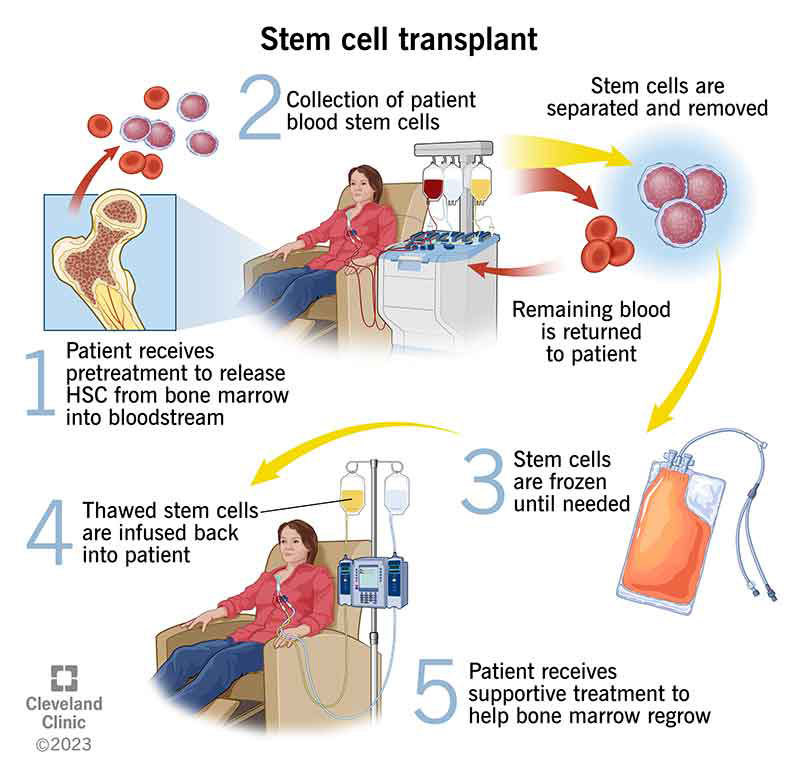
Stem Cell Transplant (Bone Marrow Transplant)

FDA approves 2 gene therapies for sickle cell disease
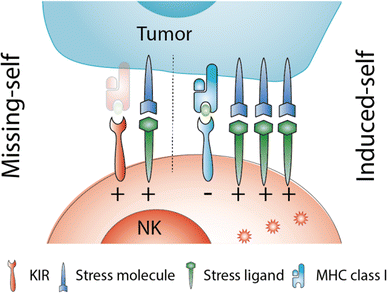
NK cell therapy after hematopoietic stem cell transplantation: can

Reprogramming stem cells in regenerative medicine - Mao - 2022
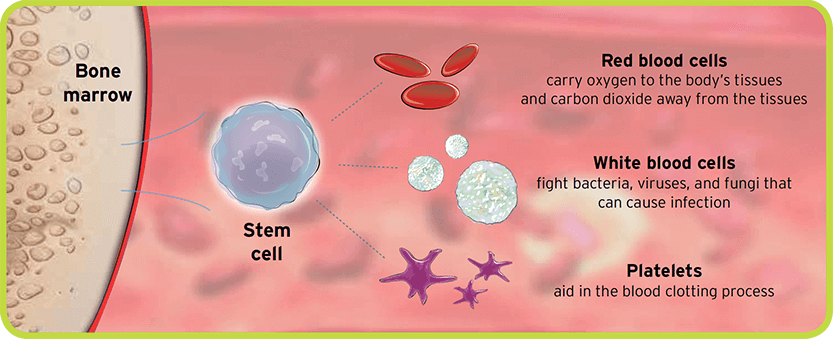
About Stem Cell Transplant Mozobil® (plerixafor) injection

Towards regulatory cellular therapies in solid organ

Mesenchymal Stromal Cells: an Antimicrobial and Host-Directed

Reprogramming stem cells in regenerative medicine - Mao - 2022

How a stem cell treatment left patients feeling worse than before

Liver Disease: Induction, Progression, Immunological Mechanisms

FDA-Approved Cell Therapy Protects Patients After Stem Cell Transplant
de
por adulto (o preço varia de acordo com o tamanho do grupo)