Statistics in Medicine — Reporting of Subgroup Analyses in
Por um escritor misterioso
Descrição

Statistics in Medicine — Reporting of Subgroup Analyses in Clinical Trials
If you spend enough time following biotech, you’ll encounter a common situation: A big clinical trial fails, but the company points to a glimmer of an

Subgroup analysis: How to evaluate post hoc tests for significance in failed clinical trials

Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data - The Lancet Diabetes & Endocrinology

Understanding of interaction (subgroup) analysis in clinical trials - Brankovic - 2019 - European Journal of Clinical Investigation - Wiley Online Library

Subgroup analyses for matched cohorts (unadjusted). P values for

Inplasy Protocol 434 – INPLASY

Randomized controlled trial - Wikipedia

Subgroup analysis, covariate adjustment and baseline comparisons

Accuracy and precision of non-invasive cardiac output monitoring devices in perioperative medicine: a systematic review and meta-analysis† - British Journal of Anaesthesia

Systematic Reviews - Exercise Science - LibGuides at Lebanon Valley College
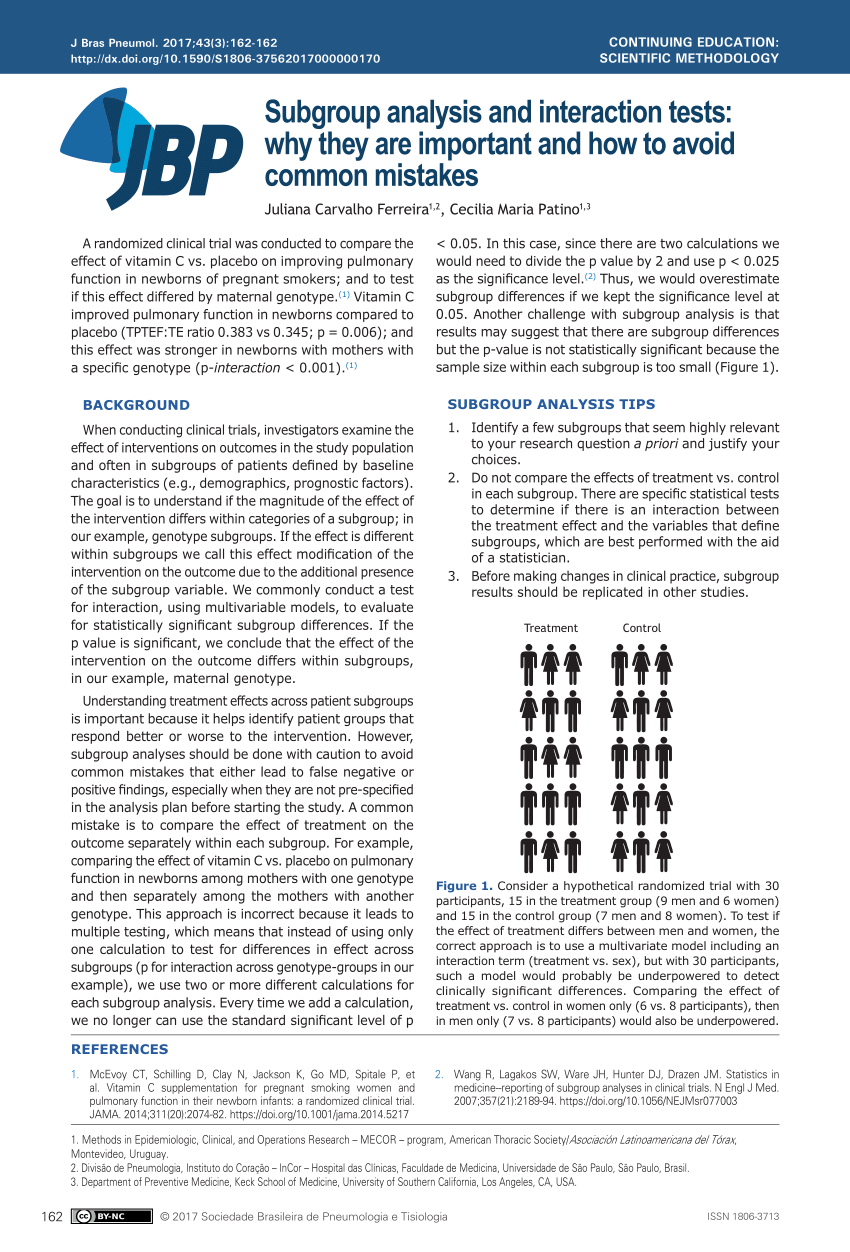
PDF) Subgroup analysis and interaction tests: why they are important and how to avoid common mistakes

About

How to interpret subgroup analyses? Insights from the statistician

Statistics in Medicine — Reporting of Subgroup Analyses in Clinical Trials
de
por adulto (o preço varia de acordo com o tamanho do grupo)





:max_bytes(150000):strip_icc():focal(999x0:1001x2)/chloe-moretz1-f4057f86611241818317921f65582cdc.jpg)